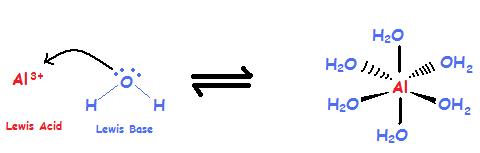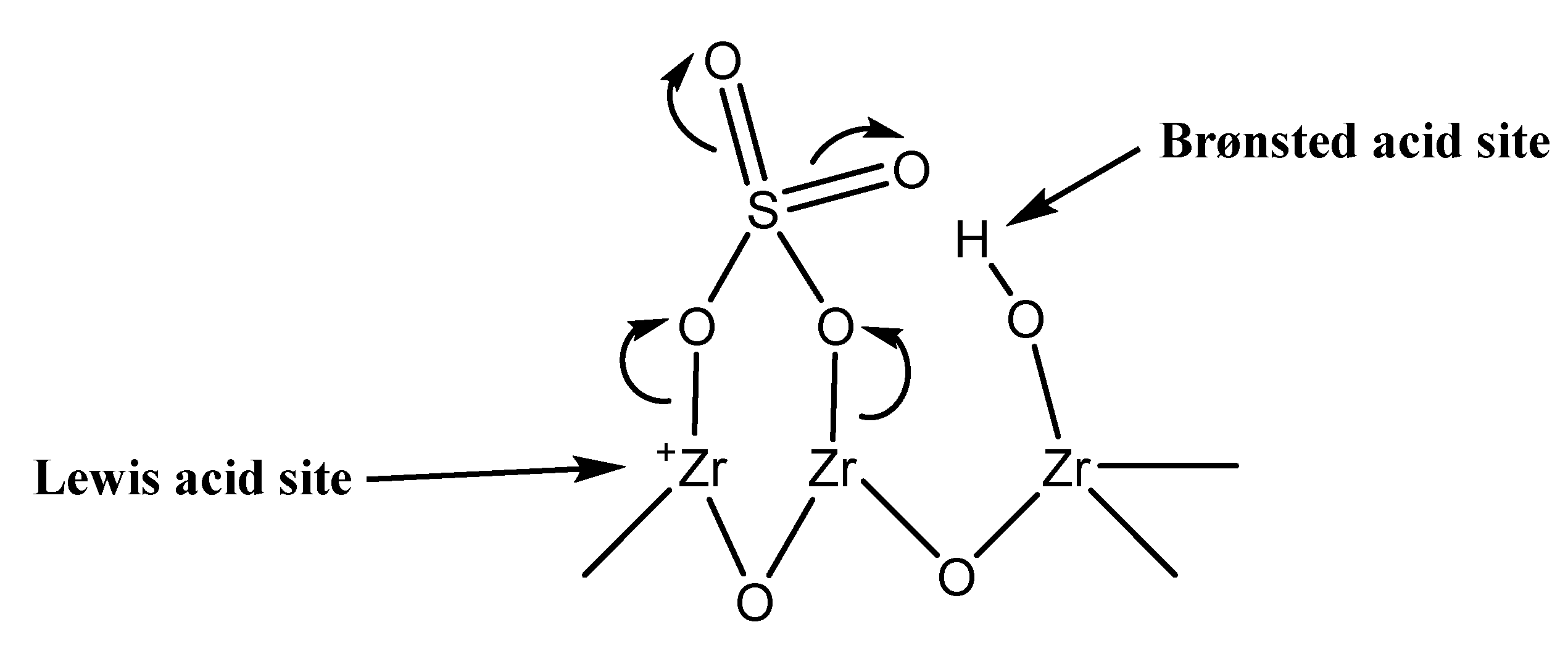
Nanomaterials | Free Full-Text | Comparative Study of Different Acidic Surface Structures in Solid Catalysts Applied for the Isobutene Dimerization Reaction

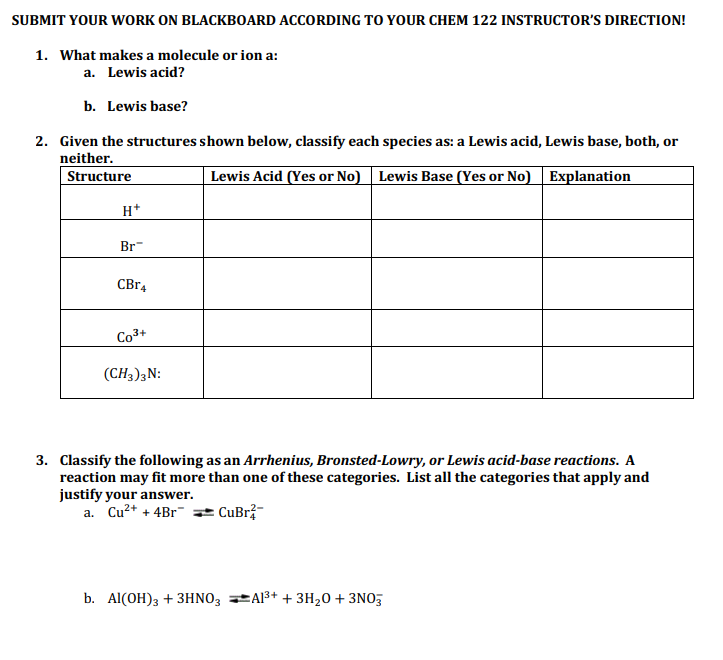
Defects passivation by Lewis acid or Lewis base. a Diagram depicting... | Download Scientific Diagram
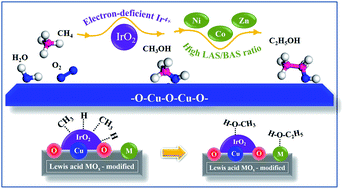
Lewis acid (Ni2+, Co2+/3+ or Zn2+) modified electron-deficient Ir4+ in IrO2/CuO for promoting methane oxidation to ethanol and methanol - Journal of Materials Chemistry A (RSC Publishing)

Ni‐Ti Intercalated and Supported Bentonite for Selective Hydrogenation of Cinnamaldehyde - Xu - ChemPhysChem - Wiley Online Library

SOLVED: Question 7 (1 point) Ht has no electron: N donates two electrons to form the bond: Latin "dative Can anything here be called a Lewis acid or Lewis from the base?
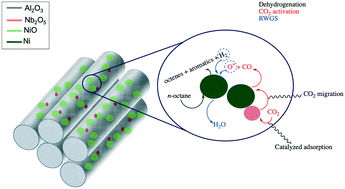
Nb2O5 as a radical modulator during oxidative dehydrogenation and as a Lewis acid promoter in CO2 assisted dehydrogenation of octane over confined 2D engineered NiO–Nb2O5–Al2O3 - Catalysis Science & Technology (RSC Publishing)

Design of Lewis Acid Centers in Bundlelike Boron Nitride for Boosting Adsorptive Desulfurization Performance | Industrial & Engineering Chemistry Research

Atomically dispersed Lewis acid sites boost 2-electron oxygen reduction activity of carbon-based catalysts | Nature Communications

Combining Ni3P and Lewis Acid–Base Pair as a High-Performance Catalyst for Amination of 1-Octanol | SpringerLink

Role of Brønsted and Lewis acid sites on Ni/TiO2 catalyst for vapour phase hydrogenation of levulinic acid: Kinetic and mechanistic study - ScienceDirect

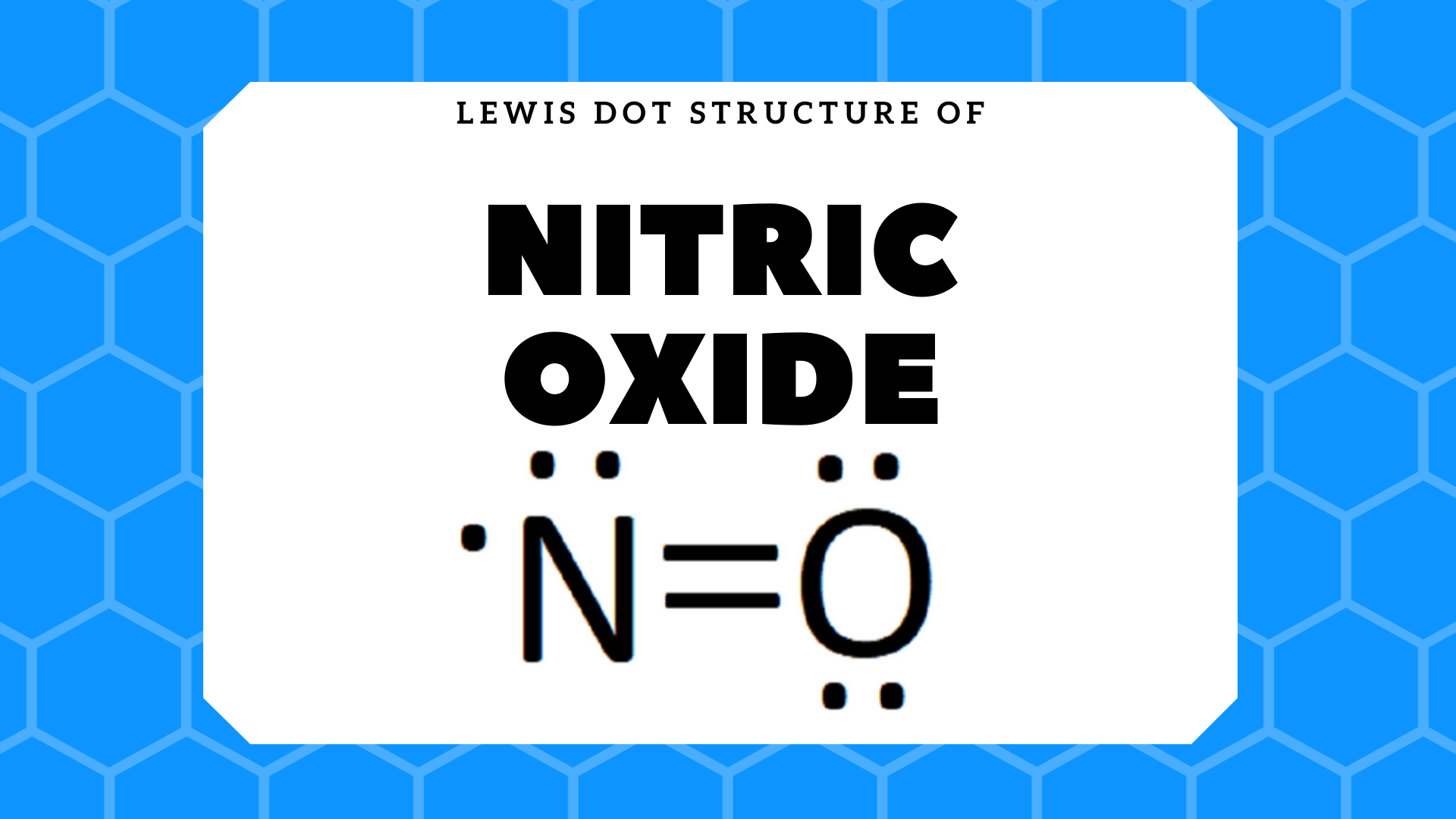
Lewis acid-assisted reduction of nitrite to nitric and nitrous oxides via the elusive nitrite radical dianion | Nature Chemistry

SOLVED: Question 12 Not vet answered Identify the Lewis Acid and Base in the reaction of Zn2+ and CN" to form Zn(CN)2: Points out of 5 Select one: a.Zn(CN)z is the Lewis