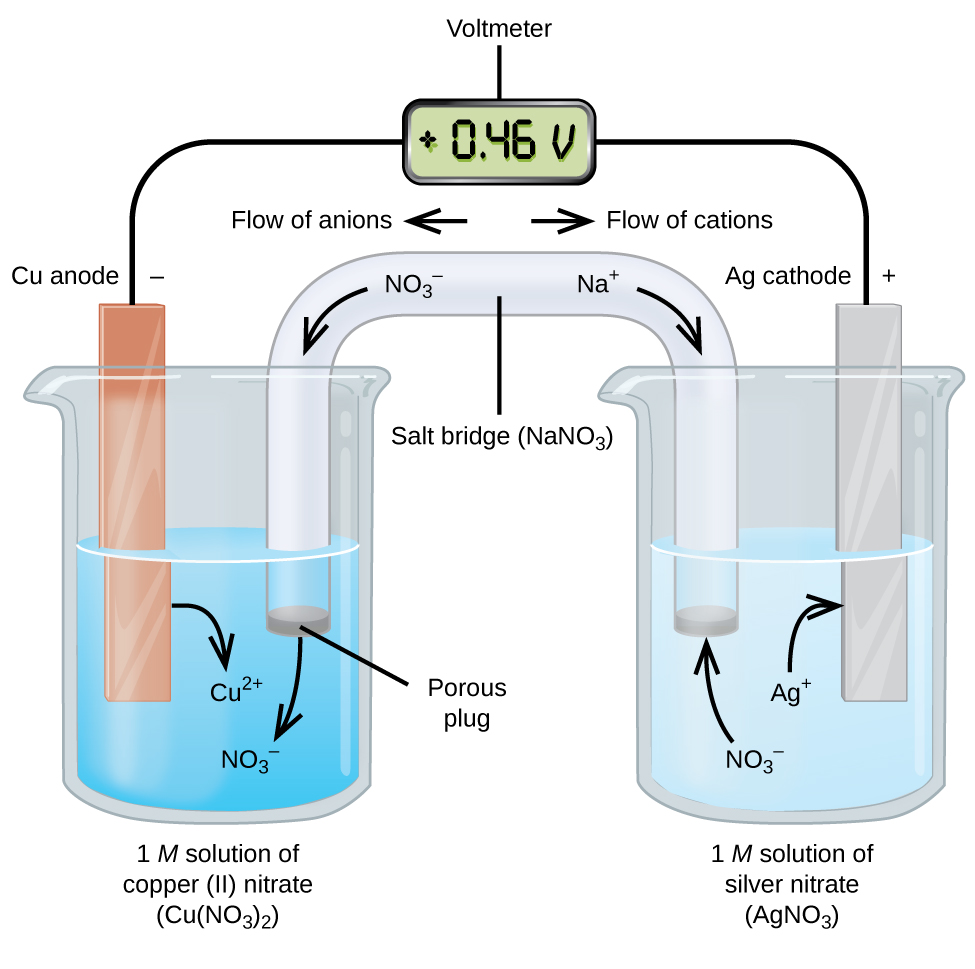
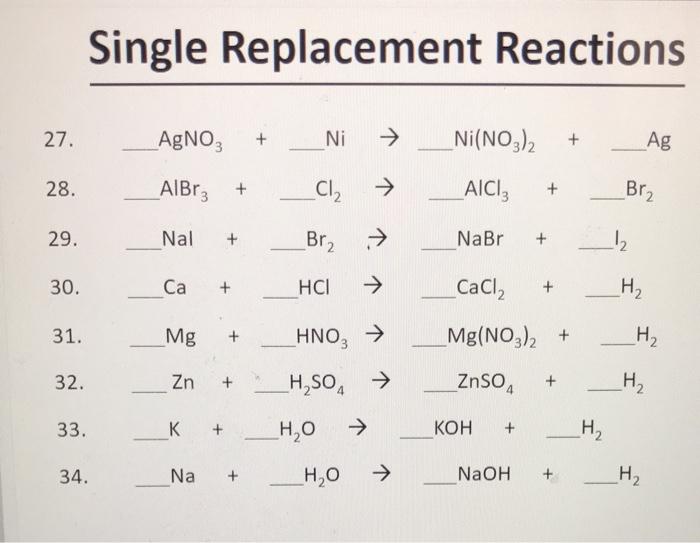
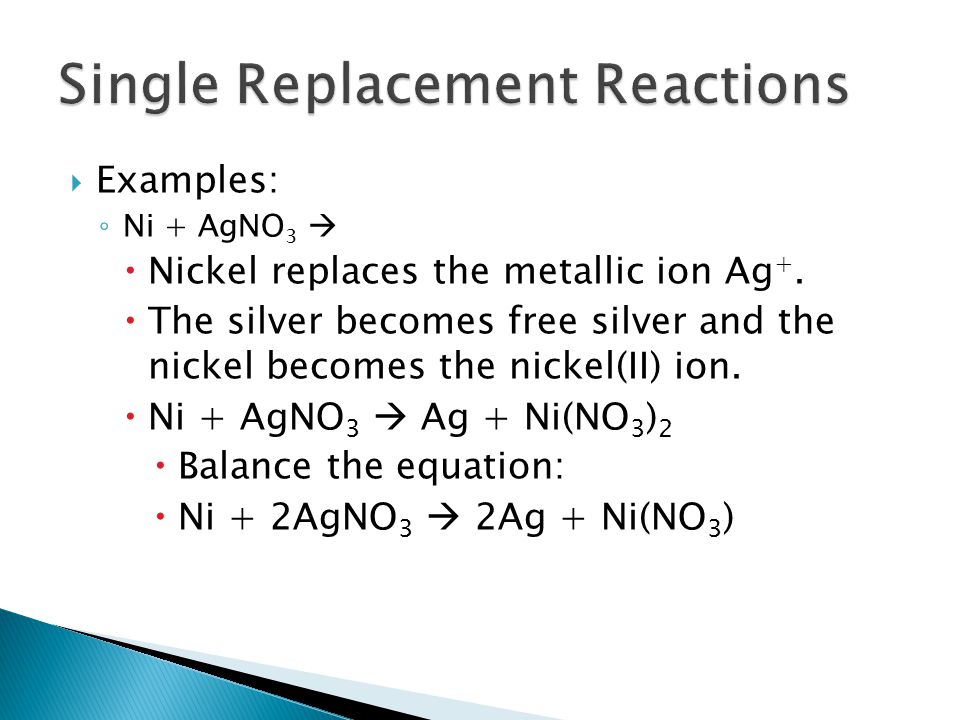
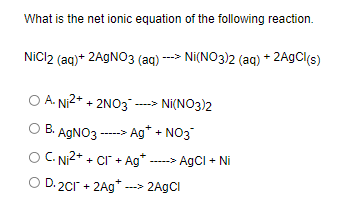A strip of nickel metal is placed in a 1 molar solution of Ni(NO3)2 and a strip of silver metal is placed in a one molar - Sarthaks eConnect | Largest Online

OneClass: check the results of your tests against the solubility rules and write balanced equation fo...

A strip of copper is placed in four different colourless salt solutions. They are: KNO3, AgNO3, Zn(NO3)2, Ca(NO3)2 . Which one of the solutions will finally turn blue?

SOLVED: Write the molecular; complete ionic, and net ionic equations for each of the following reactions K2SO4(aq) + 2 AgNO3(aq) 7 BaClz(aq) + Na2SO4(aq) Ni(NO3)2(aq) + Mg(OH)2(aq) 2 LizPO4(aq) + 3 CaClz(aq)

A strip of nickel metal is placed in a 1 molar solution of Ni(NO3)2 and a strip of silver metal - Chemistry - Electrochemistry - 9165083 | Meritnation.com