![Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni (OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni (OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com](https://homework.study.com/cimages/multimages/16/1.1.17267916333899178067.png)
Which of the coordination complexes listed below is the most stable (i.e. has the highest formation constant)? a. [NiEDTA]2- b. [Ni(CO)6]2+ c. [Ni (OH2)6]2+ d. [Ni(NH3)6]2+ e. NiCl2 | Homework.Study.com

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
![SOLVED: Consider the following Lewis acid-base reaction: Ni2+ + 6NH3 → [Ni( NH3)6]2+ Which frontier orbital on the acid will react with which frontier orbital on the base? SOLVED: Consider the following Lewis acid-base reaction: Ni2+ + 6NH3 → [Ni( NH3)6]2+ Which frontier orbital on the acid will react with which frontier orbital on the base?](https://cdn.numerade.com/ask_previews/167e7b00-77c4-444c-a59e-254dd8d41d0a_large.jpg)
SOLVED: Consider the following Lewis acid-base reaction: Ni2+ + 6NH3 → [Ni( NH3)6]2+ Which frontier orbital on the acid will react with which frontier orbital on the base?
![SOLVED: 1. Write the chemical reactions (balanced) for the following reactants. NiCl2 and HCl to produce [NiCl6]4- [NiCl6]4- and H2O to produce [ Ni(H2O)6]2+ [Ni(H2O)6]2+ and Na2EDTA to produce [Ni(EDTA)] [Ni(H2O)6]2+ and NH4OH SOLVED: 1. Write the chemical reactions (balanced) for the following reactants. NiCl2 and HCl to produce [NiCl6]4- [NiCl6]4- and H2O to produce [ Ni(H2O)6]2+ [Ni(H2O)6]2+ and Na2EDTA to produce [Ni(EDTA)] [Ni(H2O)6]2+ and NH4OH](https://cdn.numerade.com/ask_previews/9fa67892-2e91-49e4-9a3b-403dad230868_large.jpg)
SOLVED: 1. Write the chemical reactions (balanced) for the following reactants. NiCl2 and HCl to produce [NiCl6]4- [NiCl6]4- and H2O to produce [ Ni(H2O)6]2+ [Ni(H2O)6]2+ and Na2EDTA to produce [Ni(EDTA)] [Ni(H2O)6]2+ and NH4OH

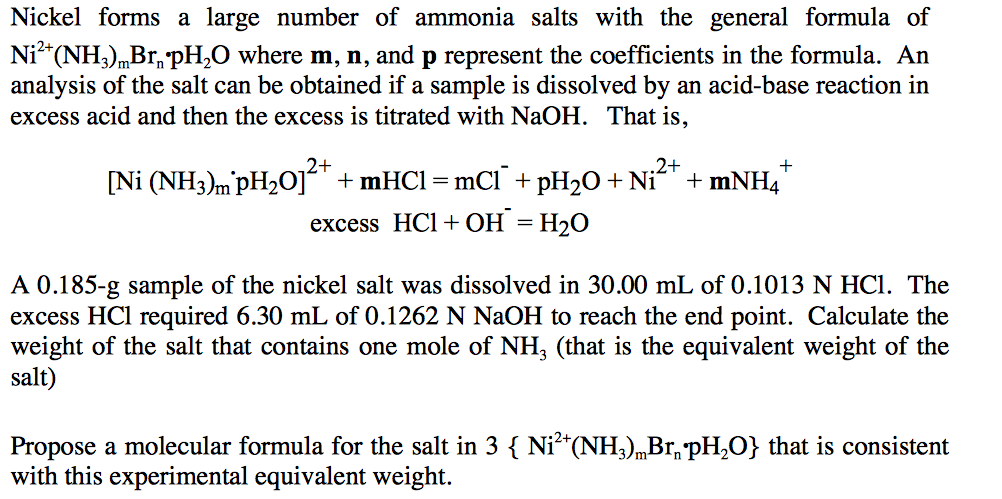
Experiment 5 –Synthesis and Stoichiometric Analysis of Hexaamminenickel (II) Chloride | SIC1002 - Inorganic Chemistry I - UM | Thinkswap
![SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale](https://cdn.numerade.com/ask_images/789c0aaf514c4932b19dafca225933f9.jpg)
SOLVED: QueSTion Consider the following = equilibrium: [Ni(H2O)6]2+ (aq) 6NHg (aq) = [Ni(NH3)]2+ (aq) 6Hz0 () Which direction does the reaction shif / by adding the HCI? QuesTion What initially fonns pale
![On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into On treatment of [ Ni (NH3)4 ]^2 + with concentrated HCl , two compounds I and II having the same formula, [ NiCl2 (NH3)2 ] are obtained, I can be converted into](https://i.ytimg.com/vi/Dx60N63HjFU/maxresdefault.jpg)
![Nanomaterials | Free Full-Text | Formation of Nanostructured Carbon from [Ni (NH3)6]3[Fe(CN)6]2 Nanomaterials | Free Full-Text | Formation of Nanostructured Carbon from [Ni (NH3)6]3[Fe(CN)6]2](https://www.mdpi.com/nanomaterials/nanomaterials-10-00389/article_deploy/html/images/nanomaterials-10-00389-g001.png)







![Answered: he absorption spectrum of [Ni(NH3)6]2+… | bartleby Answered: he absorption spectrum of [Ni(NH3)6]2+… | bartleby](https://content.bartleby.com/qna-images/question/d6205eee-3e74-4cd4-a03f-f1935e30990d/1962a177-e50e-48c0-92d6-94862abbf6cf/bdhulbi_processed.jpeg)

![Solved Or blue color like [Ni(NH3) 6]2+ (aq)? An aqueous | Chegg.com Solved Or blue color like [Ni(NH3) 6]2+ (aq)? An aqueous | Chegg.com](https://media.cheggcdn.com/study/437/437f5cd4-67bf-4a61-bbd0-59efdb460b96/image.png)